Despite advances in targeted therapy and use of molecular/cytogenetic risk stratification to guide treatment, clinical outcomes for patients with acute myeloid leukemia (AML) remain unfavorable. Harnessing the immune system with CAR-T or immune checkpoint inhibitors (ICIs) has been proven successful in other hematological malignancies and solid tumors. The use of these immunotherapies for the AML treatment remains challenging due to lack of selective target antigens or low expression of immune checkpoint proteins. Recently, one strategy proposed to transform AML from immune-cold to immune-hot is to trigger a type-I interferon (IFN-I) response via a dsDNA related cGAS/STING signaling or dsDNA related RIG-I/MDA5 signaling. We recently reported that the dNTP and Ara-CTP (active form of cytarabine [AraC]) hydrolase SAMHD1, whose expression levels are elevated in AML patients relative to normal donors, limits IFN-I and T cell responses in murine AML model ( Blood, 2022, 140 [Supplement 1]: 679-680). We also found that innate immune activation seen following SAMHD1 knockdown is associated with its catalytic activity inhibition in cancer cells, providing a rationale for the development of SAMHD1 inhibitor.
To identify SAMHD1 inhibitor, we conducted a virtual screen on 8,000,000 compounds based on SAMHD1 crystal structure (PBD 6TXC). The top 500 hits were then assessed in SAMHD1-WT- or H233A (SAMHD1 loss-of-function mutant) -expressing Molm13 cells using a phenotypic screen for AraC sensitization. Specifically, a SAMHD1 inhibitor should sensitize SAMHD1-WT (S-WT) but not H233A (S-H233A) cells to AraC treatment. Among the top 20 hits from phenotypic screen, our HPLC-based cell-free and cell-based SAMHD1 enzymatic assay revealed Samin27 as the most potent hit in blocking SAMHD1 hydrolysis and promoting AraCTP accumulation. Therefore, we assessed the efficacy of Samin27 combined with AraC in vivo. Samin27 administration (25mg/kg i.p. qd, starting on day 8 for 7 days) enhanced AraC efficacy (50mg/kg i.p. qd, starting 8th day for 5 days) in NSG mice implanted with Molm13 cells, as evidenced by extended survival relative to AraC alone. Moreover, the physical interaction between Samin27 and SAMHD1 protein were confirmed in a Carr-Purcell-Meiboom-Gill (CPMG) nuclear magnetic resonance (NMR) titration assay.
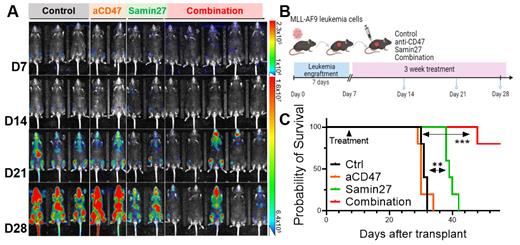
RNA-seq results revealed Samin27 ex-vivo treatment remarkably upregulated IFN-I response genes in Molm13 cells, consistent with transcriptome results of SAMHD1-knockdown (S-KD) Molm13 cells. SAMHD1 activity is known to restrict replication of LINE-1 (L1), which is the only autonomously active transposable elements (TE) in mammals; elevated retrotransposition induces DNA damage and promotes cytoplasmic DNA accumulation, resulting in an IFN-I response via cGAS signaling. Next, to explore the mechanism underlying SAMHD1-inhibition induced immunity, we asked if SAMHD1 loss promoted retrotransposition and upregulated TE transcripts in leukemia cells. Through re-analysis of RNA-seq data in Molm13 cells, we found SAMHD1 inhibition upregulated most L1 elements and LTR-containing endogenous retrovirus (ERV) subfamilies. To determine if Samin27 induced immunity via L1 upregulation, we employed 3TC treatment (3TC, the nucleoside reverse transcriptase inhibitor) to block L1 upregulation. 3TC treatment blocked Samin27-mediated IFN-I response genes upregulation. Given that IFN-I response can stimulate innate immune cells (macrophage and T cell) cross-priming, potentially rendering AML cells more susceptible to anti-CD47 monoclonal antibody (aCD47) treatment, we then assessed whether combining a SAMHD1 inhibitor with an aCD47 act synergistically. We first implanted murine MLL-AF9 (MA9) leukemia cells into WT B6 mice. Then we treated leukemic mice with isotype control (Ctrl), anti-CD47 (BE0283 [BioXCell], 10mg/kg, i.p. qd, 3 weeks), Samin27 (25mg/kg i.p. qd, 3 weeks) or combination. Notably, pharmacological inhibition of SAMHD1 remarkably inhibited leukemia progression, decreasing leukemia burden (Fig. A.B). In another cohort, combination treatment extended mouse survival (Fig. C). Collectively, our results may provide a rationale for combining Samin27 with aCD47 against AML population, where single anti-CD47 treatment shows little effects.
Disclosures
Salhotra:BMS: Research Funding; OrcaBio: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees; Sanofi: Speakers Bureau; Rigel Pharma: Research Funding; Jazz Pharma: Research Funding; Kura Oncology: Research Funding; Gilead: Research Funding. Marcucci:Ostentus Therapeutics: Current equity holder in private company, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal